1. What is an acid? – the difference between strong and
weak acids?
2. Give examples of usually used acids and what they are
used for?
3. Mention two weak acids, chemical name, properties?
4. Name three strong acids, chemical name, properties?
5. The AIW-rule
6. What is a base?
7. Mention a weak base, chemical name, properties?
8. Mention two strong bases, chemical name, properties?
9. What is neutralization, also give an example?
10. What is BTB (bromothymol blue)? What color has it in
acidic, neutral and alkaline solution?
11. How does the pH-scale work?
12. What does it mean if a solution is: dilute, concentrated
or saturated?
13. Questions
What is an
acid? – the difference
between strong and weak acids?
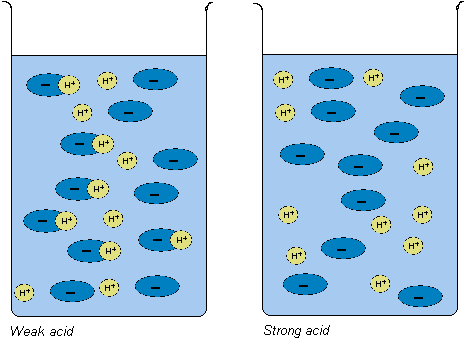
An acid is a substance that leaves off or can leave off a
hydrogen ion (H+). This means that there are free hydrogen
ions in solution. The solution thus becomes acidic. A strong
acid always leaves off a hydrogen ion and a weak acid leaves
sometimes off a hydrogen ion. This means that even a weak
acid has an acid solution, since there are free hydrogen
ions in the solution.

Acids can cause nasty burns and also dissolve metals.
Therefore, bottles of acid often have the warning symbol
"warning corrosive".
Give examples of usually used
acids and what they are used
for?
Weak acids:
Acetic acid: used for preservation of e.g. cucumber, or as
part of window cleaner.
Citric acid: used in cooking for acidification, or as part
of a preservative.
Strong acids:
Sulfuric Acid: Used as a liquid (electrolyte) in car
batteries.
Mention two weak acids, chemical name, properties?
Meta acid (formic acid): HCOOH
Use: silage crops for preservation for the winter. In this
way crops as food for animals are saved for the winter
months. Lactic acid bacteria acidify crops and preserve
them.
Formic acid is clearly noticeable when stirring around in an
anthill. The ants produce this acid as part of their defense
system. If you have a wound on your hand and stirring the
anthill the wound will sting thoroughly. (Note: be careful
and considerate towards ants - they cooperate better than
most humans - watch and learn).
Ethanoic acid (acetic acid): CH3COOH
Pure acetic acid solidifies already at a temperature of 17
oC. The acid can be produced in various reactions, e.g.
through dry distillation of wood. Transforming action of
certain bacteria in alcohol (ethanol) can also produce
acetic acid.
Name three strong acids, chemical name, properties?
Hydrochloric acid: (HCl)
HCl is actually a gas. It dissolves in water and
hydrochloric acid is formed. Hydrochloric acid often fumes
as a result of the acid leaving the liquid as gas. The gas
can once again form small droplets of hydrochloric acid.
Hydrochloric acid has a pungent characteristic odor. The
hydrochloric acid reacts with water as follows:
HCl  H+ + Cl-
H+ + Cl-
The liquid contains free hydrogen ions and free chloride
ions.
Sulfuric acid: (H2SO4)
Sulfuric acid is an odorless acid with a fairly high
density. It may seem slightly viscous. A noteworthy feature
is that the acid adds water. Therefore, it has been used for
adding moisture between glasses in windows. The container
with acid has to be periodically emptied, since the amount
of fluid is constantly increasing, and could otherwise
overflow the container.
Nitric acid: (HNO3)
Nitric acid is stored in the dark, as it would otherwise
decompose by sunlight. The acid has the ability to dye
proteins yellow. This is the case if placing a droplet of
nitric acid on a nail. After a while, the area is dyed
yellow. When the acid reacts with base metals gases called
nitrogen oxides are emitted. These are reddish brown and
very toxic.
The AIW-rule
When you mix acid and water you should always pour the acid
into the water. The reason is that heat develops when mixing
these two substances. If you pour water into acid, a water
droplet entering the acid will immediately get very hot and
it can turn into a gas. Should this happen there is the risk
of the droplet splashing up bringing some acid. This
splashing can surprise the person who is mixing (bumping).
If instead pouring acid into water the risk is avoided.
What is a base?
A base is a substance that can add a hydrogen ion. An
example of a base is ammonia (NH3). When ammonia is poured
into water, ammonia reacts with water adding a hydrogen ion
taken from a water molecule. Left of the broken water
molecule are one oxygen atom and one hydrogen atom joined as
a hydroxide ion (OH-). In a solution of a base there are
always hydroxide ions.

Bases dissolve fat and have therefore the safety alert
"warning corrosive".
Mention a weak base, chemical name,
properties?
Ammonia is a weak base since only part of the ammonia
molecules reacts with water molecules and adding (absorbing)
hydrogen ions, leaving behind hydroxide ions. Below is an
example of an ammonia molecule reacting with a water
molecule:
NH3 + H2O 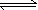 NH4+ + OH-
NH4+ + OH-
The bidirectional arrow indicates that not all ammonia
molecules react with water molecules. The reaction gives
rise to free hydroxide ions in solution, but there are also
ammonia molecules that have not reacted with water
molecules. The fact is that the reaction goes both
directions.
Mention two strong bases, chemical name, properties?
Strong bases function in a slightly different way. Strong
bases are mostly salts.

The above picture shows crystals of sodium hydroxide. The
salt looks like small flakes.
A salt dissolves in water into positive and negative ions.
The base sodium hydroxide (NaOH) will release sodium ions
(Na+) and hydroxide ions (OH-). This happens according to
the reaction formula:
NaOH  Na+
+ OH-
Na+
+ OH-
The hydroxide ion makes the solution alkaline. All hydroxide
ions are released from the sodium ions. We say that the base
is strong.
Another example of a strong base is potassium hydroxide.
Potassium hydroxide is dissolved in water as:
KOH  K+ + OH-
K+ + OH-
What is neutralization, also give an example?
A neutralization takes place if an acid reacts with a base.
Example:
HCl + NaOH  Na+
+ Cl- + H2O
Na+
+ Cl- + H2O
Sodium ions and chloride ions are free in solution. Ordinary
saline water remains. Hydrogen ions and hydroxide ions react
as follows:
H+ + OH-  H2O
H2O
Neutralization has occurred. The acid has neutralized the
base or vice versa, i.e. the base has neutralized the acid.
What is BTB (bromothymol blue)? What color has it in acidic,
neutral and alkaline solution?
An acid-base-indicator indicates whether a solution is
acidic or alkaline. BTB (bromothymol blue) is a common
indicator.
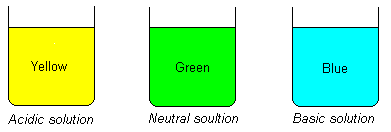
How does the pH-scale work?
pH < 7 : acid solution. Here are hydrogen ions (H+)
pH = 7 : neutral solution
pH > 7 : alkaline solution. Here are hydroxide ions (OH-)
![]()
What does it mean if a
solution is: dilute, concentrated or
saturated?
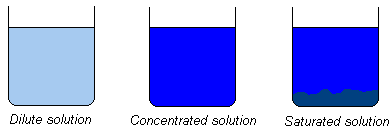
A dilute solution has just a small amount of salt dissolved.
In a concentrated solution there is so much salt dissolved,
that a little bit of extra salt would not be able to
dissolve, but instead form an insoluble heap on the bottom.
If that happens, the solution is called saturated.
Questions
1.
What is an acid?
2.
Which ion does an acid give off?
3.
Mention a weak acid?
4.
Mention a strong acid?
5.
What is the difference between a weak and a strong acid?
6.
What is the chemical symbol of methane acid?
7.
What is formic acid used for?
8.
What is the chemical name of ethanoic acid?
9. What is the popular name of ethanoic acid?
10.
What is ethanoic acid used for?
11.
Give the chemical symbol of ethanoic acid?
12.
Give the chemical symbol of sulfuric acid?
13.
Give the chemical symbol of hydrochloric acid?
14.
Give the chemical symbol of nitric acid?
15.
Show how hydrochloric acid dissolves in water - with
chemical symbols?
16.
What do the letters in the AIW-rule stand for?
17.
What is the AIW-rule - what does it mean?
18.
Define a base – what is a base?
19.
What ion is present in an aqueous solution of a base?
20.
Mention a weak base with its name and chemical name?
21.
Show the chemical reaction formula of this weak base
dissolves in aqueous solution?
22.
Mention a strong base with name and chemical name?
23.
Show how this strong base dissolves in water?
24.
What is the difference between a weak and a strong base?
25. What is neutralization?
26.
Show with chemical symbols how hydrochloric acid neutralizes
sodium hydroxide?
27.
What is bromthymol blue (BTB)?
28.
What color has BTB in acidic, neutral and alkaline solution?
29.
Tell about the pH-scale?
30.
What is the meaning of a low pH?
31.
What is the meaning of a high pH?
32.
What is a dilute solution?
33.
What is a concentrated solution?
34.
What is a saturated solution?
Copywrite NGU, Northern Pontifical Academy 2025 (A.I.C.)