1. Describe the three ways of heat transfer?
2. The ability of material surfaces to absorb thermal
radiation
3. Thermal conduction in different materials
4. What happens when a material is heated? What is rail
buckling?
5. Thermometer
6. Boiling point
7. Boiling
8. Condensation
9. Melting
10. Solidification
11. Freezing point
12. Evaporation
13. Try using the particle approach (solid – liquid –
gaseous)
14. Distillation
15. What did the scientists Celsius, Fahrenheit and Kelvin
do?
Describe the three ways of
heat
transfer?
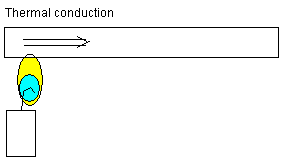
Thermal conduction: The atoms in the metal material above
the candlelight are added heat energy. The atoms start
vibrating more and more. The vibrations are spread to
neighbor atoms. Heat energy conducts from atom to atom
farther to the right.
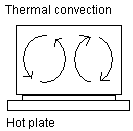
Thermal convection: Here is shown a saucepan with water on a
hot plate. The hot plate transports heat through thermal
conduction to the bottom of the saucepan. When the water is
heated, the activity increases in the water molecules. The
water molecules need more space and the density of the water
decreases. This makes the water lighter and it floats
upwards. Once the water reaches the surface it cools off and
the water once again becomes denser and contracts and sinks
towards the bottom. Convection cells are formed.
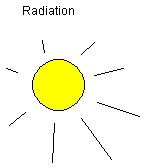
Thermal radiation: Heat can be transported through
radiation, electromagnetic waves. The material hit by
radiation absorbs part of the radiation and the material
gets hot. Some materials reflect most of the radiation, e.g.
a mirror. Dark surfaces absorb much of the radiation energy.
The type of radiation also matters. Thermal radiation is
included in visible light.
This is the only heat transfer method possible through
vacuum or space. Thermal conduction and thermal convection
both need a medium, i.e. matter to travel with or through.
The ability of material surfaces to
absorb thermal radiation
The rougher the surface is, the greater the ability of the
material to absorb thermal radiation and visible light.
Black color is a natural radiation trap. When energy is
absorbed by the color no radiation is emitted in the visible
spectrum, i.e. radiation that we can see with our physical
eyes.
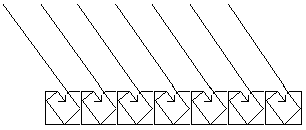
No radiation in the visible spectrum leaves the surface.
Energy will anyway leave the surface. This energy is emitted
as infrared radiation. We cannot see this radiation, but we
can experience it as heat.
Thermal conduction in different materials
If the material conducts heat well, like iron or copper,
absorbed heat can efficiently be transported through the
material. Metal atoms share electrons that quickly transfer
energy to neighbor metal atoms. In contrast to iron and
copper, wood cannot conduct heat well. The material will
instead get warm on the surface – the surface that is
heated. Materials such as wood insulate well. You can walk
on glowing coal, since it takes relatively long time for the
coal to conduct heat into the foot.
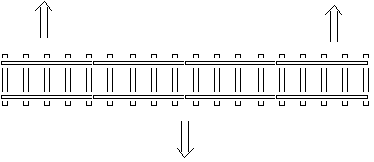
What happens when a material is heated? What is
rail
buckling?
When a material is heated, it takes up more space and
expands. A piece of iron that gets warmer thus takes more
room. An example is when rail men adds rail to a railway. If
they build the railroad in the winter and add rail sections
tight together, then in the summer when the rail irons are
heated by the sun, the rails will take up more space and
expand. The iron-rail-bars thus become longer, and if no
space exists between the rail bars, this additional
extension will be taken out laterally, allowing the rail
bars to curve. The entire railway line will be curved.
Thermometer

A thermometer often consists of a thin glass tube with
closed bottom. When the temperature raises the fluid inside
expands and rises indicating a higher temperature. Not long
ago mercury liquid was used, and nowadays alcohol is the
fluid. Both of these liquids are fluent even at temperatures
below zero. Water cannot be used in freezing conditions
since water freezes to ice at 0 °C. Ice needs more space
than its liquid state. This could cause the surrounding
glass to break. Water is almost the only substance that has
a greater volume in solid state compared to its liquid
state.
Boiling point,
Boiling, Condensation, Melting, Solidification, Freezing
point, Evaporation
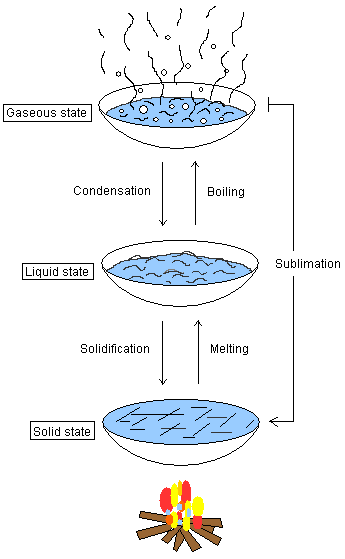
Boiling point
The boiling point is the temperature at which the substance
changes from liquid to gaseous state. The gas pressure in
the water (water molecules) is the same as the atmospheric
gas pressure. The boiling point in water is 100 oC.
Boiling
A liquid turns to its gaseous state. See boiling point
above.
Condensation
Condensation means that a gas turns to its liquid state. An
example is when water vapor encounters a mirror in a
bathroom. The mirror takes up heat energy from the
water-gas-molecules, which means that the gas turns to
liquid. Water droplets accumulate onto the mirror.
Melting
Melting is when the solid phase of a substance turns into
its liquid state. An example is when iron is heated and
becomes liquid.
Solidification
Solidification is the opposite of melting. A liquid loses
energy and turns to its solid state, e.g. when water freezes
into ice.
Freezing point
This is the temperature at which solidification occurs.
Water solidifies at 0 oC.
Evaporation
For example, in water there are always some water molecules
having more energy than the average molecule. A water
molecule may have taken up enough energy so it can evaporate
out of a water pond. This evaporation occurs at the surface.
The water remaining in the pond has thus lost some energy,
so it has a slightly lower temperature very locally. This
lower temperature will probably not be measurable with a
standard thermometer.
Try using particle approach
Particle thinking implies envision atoms or molecules in
front of you. When heat or energy is supplied, you envision
the atoms e.g. iron atoms vibrating more and more. The
transition to liquid phase means that atoms start moving
past each other. When even more energy is added the atoms
begin to leave the solution and escape as gas.
The melting point of iron is 1538 °C and its boiling point
is 2861 oC. If wood is heated to 300 oC, it begins instead
to burn. This is because the wood fibers begin to react with
oxygen and fires up.
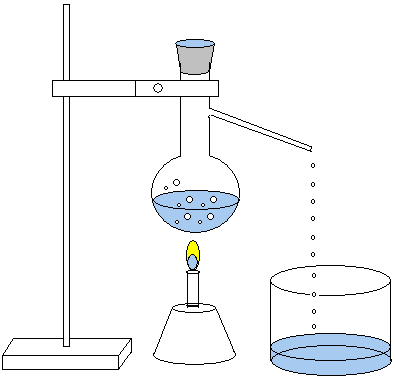
Distillation
Distillation distinguishes substances with different boiling
points. If common salt (NaCl) is dissolved in water, these
two substances can be separated through distillation. Water
has the boiling point 100 ºC. At this temperature water
boils away and turns to its gaseous state. The salt left in
the beaker has a much higher boiling point, approx. 1473 °C.
The water gas (vapor) is then made to condense when its
energy is diverted. Below is a set up of a distillation.
What did the scientists Celsius, Fahrenheit and Kelvin do?
Anders Celsius
Lived from 1701 to 1744. Has developed a temperature scale
where 0 °C was the water boiling point (also condensation
point) and 100 °C was the melting point (also freezing
point) of water. His disciple Marten Strömer later changed
the scale so it corresponds to the one we use today. Anders
Celsius was an astronomer and physicist who worked in
Uppsala. He e.g. studied the flattening of the earth’s
poles.
Fahrenheit
Lived from 1686 to 1736. He was a German physicist who
created a temperature scale used by the older
English-speaking generations. 0 F coincides with the
temperature of water and ice, when ammonium chloride is
poured in. The melting point of ice is +32 F and its boiling
point is +212 F. Here is the mathematic formula explaining
the relationship between Fahrenheit degrees and Celsius
degrees: F = 32 + 8.1C (C = degrees Centigrade).
Kelvin
Lived from 1824 to 1907. He was a British physicist. The
unit of absolute temperature (Kelvin - K) is named after
him. The scale starts from absolute zero, which is set to 0
K. Here the atoms are close together and locked in their
positions not showing especially strong vibrations. All
temperatures above 0 K means that the atoms vibrate more and
more until they have so much energy that they move past each
other and finally escape one another. The water melting
point equals 273 K and its boiling point corresponds to 373
K.
Copywrite NGU, Northern Pontifical Academy 2025 (A.I.C.)